AG Lie
Professorship of Molecular Medicine focusing on Molecular Imaging
Group Leader:
Prof. Dr. Dieter Chichung Lie
Institut für Anatomie
Lehrstuhl für Mikroskopische Anatomie und Molekulare Bildgebung
- Telefon: +49 9131 85-22264
- E-Mail: chi.lie@fau.de
Research Focus: Stem Cells and Neurogenesis in Health and Disease
During adulthood, a number of organs such as liver, gut, skin and blood rely on continuous stem cell activity for tissue homeostasis and repair. In the adult brain, stem cell activity is limited to a few select niches including the dentate gyrus (DG) of the hippocampal formation. Here, neural stem cells generate functional neurons throughout life in a process termed adult hippocampal neurogenesis. Adult hippocampal neurogenesis is essential for hippocampal information processing and is associated with a number of neuropsychiatric disorders, such as cognitive aging and depression.
Work in our laboratory aims to identify the molecular and cell biological mechanisms controlling adult neurogenesis and neurogenesis-dependent plasticity. Current projects focus on understanding the role of metabolism and cellular homeostasis in stem cell maintenance and on the identification of molecular pathways controlling the maturation and functional integration of adult-generated hippocampal neurons. We are tackling these questions using an integrated in vivo and in vitro approach utilizing a combination of techniques in molecular and cell biology, biochemistry, epigenetics, imaging, and mouse genetics.
In a second line of research our laboratory models the impact of neuropsychiatric disease genes on neurodevelopment using human pluripotent stem cells and CRISPR/Cas9 mediated genome-editing. Here, we focus on elucidating the function of transcription factors associated with intellectual disability, autism and schizophrenia on cortical neurogenesis.
Recent key publications:
Schaffner, I., Minakaki, G., Khan, M.A., Balta, E.A., Schlotzer-Schrehardt, U., Schwarz, T.J., Beckervordersandforth, R., Winner, B., Webb, A.E., DePinho, R.A., Paik, J., Wurst, W., Klucken, J. & Lie, D.C. (2018) FoxO Function Is Essential for Maintenance of Autophagic Flux and Neuronal Morphogenesis in Adult Neurogenesis. Neuron, 99, 1188-1203.
Jung, M., Haberle, B.M., Tschaikowsky, T., Wittmann, M.T., Balta, E.A., Stadler, V.C., Zweier, C., Dorfler, A., Gloeckner, C.J. & Lie, D.C. (2018) Analysis of the expression pattern of the schizophrenia-risk and intellectual disability gene TCF4 in the developing and adult brain suggests a role in development and plasticity of cortical and hippocampal neurons. Molecular Autism, 9.
Beckervordersandforth, R., Ebert, B., Schaffner, I., Moss, J., Fiebig, C., Shin, J., Moore, D.L., Ghosh, L., Trinchero, M.F., Stockburger, C., Friedland, K., Steib, K., von Wittgenstein, J., Keiner, S., Redecker, C., Holter, S.M., Xiang, W., Wurst, W., Jagasia, R., Schinder, A.F., Ming, G.L., Toni, N., Jessberger, S., Song, H.J. & Lie, D.C (2017) Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron, 93, 560-576.
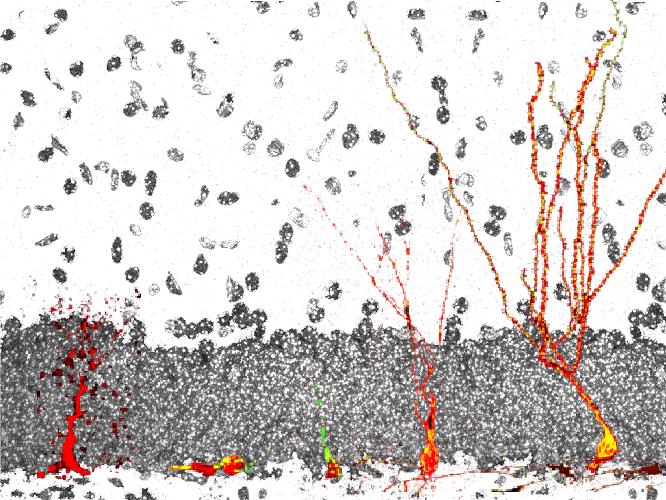
Schematic representation of the development of a neural stem cell (left) into a functional dentate granule neuron (right) in the adult hippocampal dentate gyrus.
The discovery of adult neurogenesis, i.e., the lifelong generation of new hippocampal and olfactory bulb neurons from stem cells, has added a new layer of complexity to our understanding of the mechanisms underlying plasticity and regeneration in the mammalian central nervous system. There is now strong evidence that adult neurogenesis significantly contributes to hippocampus-dependent learning and memory processes as well as to the pathophysiology of cognitive and affective symptoms during ageing and in neurodegenerative and neuropsychiatric diseases. Thus, understanding of the mechanisms regulating adult neurogenesis is of major basic neuroscientific and clinical interest. Our laboratory is tackling these questions using an integrated in vivo and in vitro approach utilizing a combination of techniques in molecular and cell biology, biochemistry, epigenetics, imaging, and mouse genetics.
Transcriptional programs in the regulation of adult neurogenesis
The generation of new functional neurons from stem cells is a complex developmental program involving activation, proliferation, fate determination maturation and selection processes. We study, how signals derived from the hippocampal microenvironment coordinate transcription factor networks and genetic programs to ensure the proper execution of the neurogenic program. Our work contributed to the identification of essential niche-derived signals and niche-induced signaling-pathways such as Wnts, Notch, and GABA/CREB-signaling in the regulation of adult neurogenesis.
In recent work we identified SoxC proteins as pleiotropic transcriptional regulators of adult neurogenesis. Our data indicate that SoxC factors not only control neuronal fate determination of neural stem/progenitor cells, but regulate survival, timing of maturation and plasticity of adult-born neurons. In ongoing projects we are investigating the regulatory pathways controlling SoxC factor expression and function as well as interacting pathways.
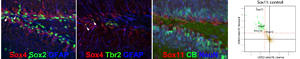
The SoxC factors Sox4 and Sox11 are highly expressed in immature adult generated neurons of the mouse hippocampus. Mass spectrometry reveals Protein Kinase A pathway as an upstream modulator of Sox11 activity. For further details see Mu et al. 2012; Balta et al. 2018a and b.
Metabolic control of stem cell development and adult neurogenesis
In contrast to adult neural stem cells, neurons are postmitotic, have a highly complex morphology and communicate with each other via high-energy consuming mechanisms. It is assumed that the generation of a functional neuron from a stem cell is accompanied by profound changes in cellular metabolism. We now found that specific mitochondrial metabolic pathways control distinct steps in neuronal development. Thus, we demonstrated that the generation of highly proliferative precursor cells from stem cells is critically dependent on electron transport chain function and oxidative phosphorylation. Interestingly, inhibition of these metabolic pathways reproduced multiple hallmarks of ageing in hippocampal neurogenesis, whereas pharmacological enhancement of mitochondrial function ameliorates age-associated neurogenesis defects. Together with the finding of age-associated alterations in mitochondrial function and morphology in neural stem cells, our data suggest mitochondrial function as a potential target to ameliorate neurogenesis-defects in the ageing hippocampus. Current work focuses on understanding, how metabolic programs are coordinated with the development of stem cells into functional neurons.
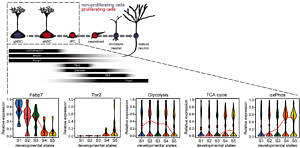
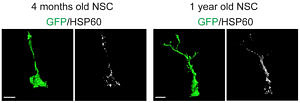
In vivo transcriptome analysis indicates a metabolic shift towards OxPhos upon generation of a highly proliferative intermediate precursor cell (IPC) from an activated stem cell (aNSC). Reconstruction of the mitochondrial compartment reveals increased mitochondrial load and altered morphology in stem cells older mice. For further details see Beckervordersandforth et al. 2017.
Autophagy in stem cell function and adult neurogenesis
Degradation and recycling of dysfunctional cellular components are critical pathways for cellular homoestasis. In particular, somatic stem cells are highly dependent on degradation and recycling pathways to maintain their lifelong capacity for regeneration. We now demonstrated that the longevity associated transcription factors of the FoxO family are critical to regulate autophagy, i.e., a central pathway for proteins and organelles, in adult neural stem cells. Loss of FoxOs does not only impair activity of the autophagic pathway but is associated with stem cell dysfunction and impaired integration of adult-born neurons. In ongoing projects we are now investigating if and how FoxO dysfunction may contribute to neural stem cell and neurogenesis dysfunction during ageing.
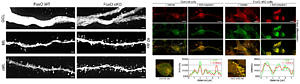
Deletion of FoxOs perturbs formation of synaptic spines. FoxO-deficient adult stem cell-derived neurons show impaired autophago-lysosomal flux. For further details see Schäffner et al. 2018.
An increasing body of evidence indicates that the pathophysiology of neuropsychiatric and -degenerative disorders may be linked to perturbation of neurodevelopmental processes. But how exactly does neurodevelopment affect disease development in later life? Does neurodevelopment determine the individual’s disease susceptibility and resilience? Does the life-long activity of neurodevelopmental processes affect onset of disease? Do neuropsychiatric and –degenerative disease genes have a developmental function?
We use human pluripotent stem cells and CRISPR/Cas9 mediated genome-editing and transgenic mouse models to study the neurodevelopmental function of genes, whose mutations have been associated with both neurodevelopmental disorders and neuropsychiatric / neurodegenerative diseases. These studies are conducted in close collaboration with researchers in the DFG-funded Research Training Group 2162 „Neurodevelopment and Vulnerability of the Central Nervous System“.
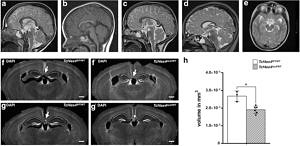
Pitt-Hopkins Syndrome-associated brain anomalies in TCF4-haploinsufficient mice. MRI shows hypoplastic corpus callosum in Tcf4-haploinsufficient PTHS patients at the age of 3 years (b) and 7 years (d). a, c Age-matched references. e Small hippocampus in PTHS patient at the age of 7 years. Tcf4-haploinsufficient mice (f’, g’) show agenesis of the corpus callosum and reduced hippocampal volume (h) compared to WT mice (f, g). For further details see Jung et al. 2018.