Other research focuses
Lehrstuhl für Biochemie und Molekulare Medizin
Group Leader:
Prof. Dr. Anja Katrin Boßerhoff
Institut für Biochemie
Lehrstuhl für Biochemie und Molekulare Medizin (Prof. Dr. Bosserhoff)
- Telefon: +49 9131 85-24190
- E-Mail: anja.bosserhoff@fau.de
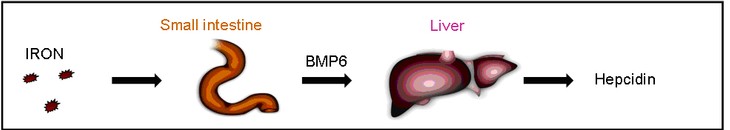
BMPs (Bone Morphogenetic Proteins) are known as modulators of iron homeostasis in the liver. BMPs are induced due to an elevated iron uptake. Via BMP receptors and the BMP-coreceptor HJV (Hämojuvelin), the hepcidin expression increases to limit the intestinale iron uptake. Our analysis revealed BMP6 as the main regulator of hepcidin. Additionally, we could show that BMP6 is expressed in the small intestine according to the iron level and is transported via the bloodstream to the liver to regulate the expression of hepcidin (Arndt et al., 2010 Gastroenterology).
Furthermore, BMPs play a major role in many cancer diseases. In our studies we could show that activation of the BMP specific smad signaling cascade is involved in progression of hepatocellular carcinoma (HCC) (Maegdefrau et al., 2012 Exp Mol Pathol).
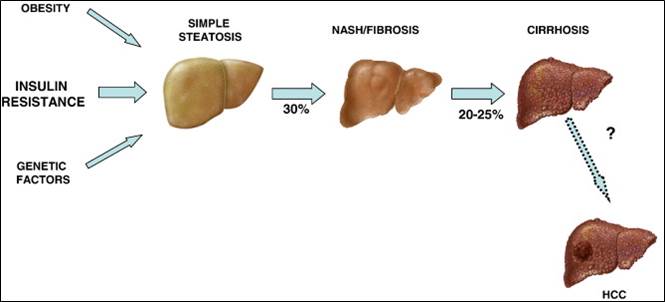
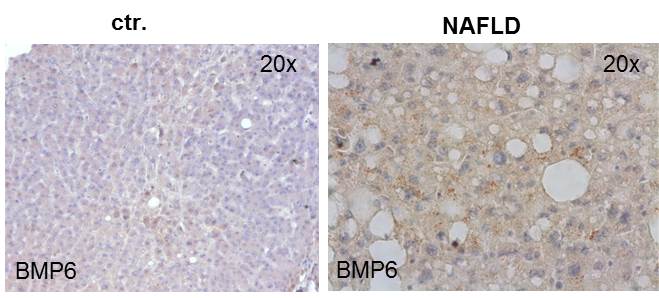
In current studies we investigate the function of BMPs in NAFLD (non-alcoholic fatty liver disease), because research on NASH (non-alcoholic steatohepatitis) mouse models and NAFLD patients showed an increased BMP6 expression in the damaged liver.
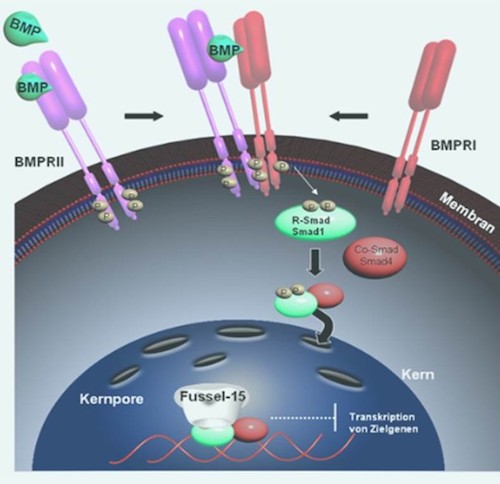
Bone Morphogenetic Proteins (BMPs) belong to the TGF-ß super family, whose members are involved in almost every cellular process. During wound healing, TGF-ß stimulates fibroblasts to migrate into the injured area, where they proliferate and effectuate the contraction of the wound margins enabeling wound closure. Moreover, fibroblasts secrete matrix components such as collagen and fibronectin to allow tissue contraction and remodeling.
We were the first to show that the Fussel-15 expression is induced during early wound healing and capable of negative regulation of BMP signaling. Fussel-15 interacts directly with the Smad molecules and inhibits thereby the transcription of several target genes.
Keloid patients, who show excessive scarring and patients with localized scleroderma, who suffer from hardening of the connective tissue, show altered Fussel-15 expression and accordingly defective TGF-ß/BMP signaling.
In the course of our studies we try to clarify the molecular function of Fussel-15 and BMPs during normal wound healing and moreover during pathophysiological processes.
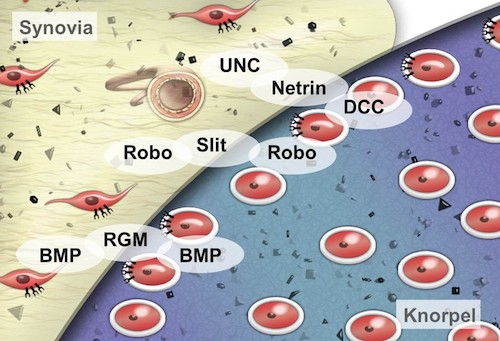
The family of repellent factors was first described in neuronal development (axon guidance). We focus on the analysis of different cell types of the synovial tissue, e.g. chondrocytes, synovial fibroblasts and human mesenchymal stem cells (hMSCs).
Here, we aim to define the role of Netrins and Slits and their receptors uncoordinated locomotion/Deleted in Colorectal Carcinoma (UNC/DCC) and roundabout (Robo) in inflammatory and degenerative alterations in the articular cartilage in rheumatoid arthritis (RA) and osteoarthritis (OA). Our studies suggest that deregulation of these molecules leads to a loss of defined tissue boundaries which promotes the progression of RA and OA. In order to indentify regulatory mechanisms of the repellent receptors the expression of miRNAs was determined. This showed a de-regulation of certain miRNAs, which regulate a variety of genes beside UNC and Robo, providing new possible targets for RA/OA therapy.
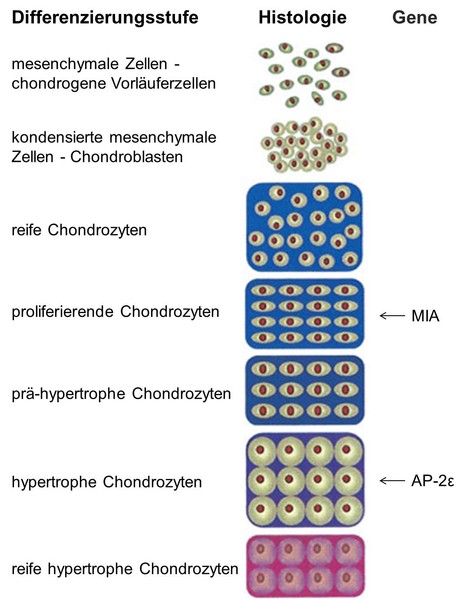
MIA, also known as CD-RAP (cartilage-derived retinoic acid-sensitive protein), is not only expressed in melanoma cells but also in chondrocytes. Analysis of the MIA/CD-RAP-knockout-mouse revealed that MIA/CD-RAP influences interactions between chondrocytes and their surrounding extracellular matrix, inhibits the proliferation of mesenchymal cells and promotes chondrocyte differentiation. Moreover we found enhanced cartilage regeneration in MIA/CD-RAP-knockout-mice during osteoarthritis and fracture healing.
Characterization of the molecular mechanism how MIA/CD-RAP effects chondrocyte differentiation is one of the major goals in this project.
As well as MIA/CD-RAP the transcription factor AP-2ɛ participates in chondrocyte maturation. We could show that AP-2ɛ promotes differentiation of cartilage in late, hypertrophic stages. The next task is to analyze the specific function of the protein during endochondral ossification and which target genes are regulated by this transcription factor.
Additionally AP-2ε plays a role in osteoarthritis, a degenerative joint disorder, and is over-expressed in osteoarthritic cartilage. Therefore the function of AP-2ɛ in the context of this disease needs to be further investigated.
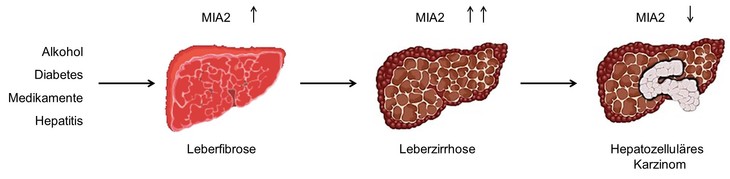
The protein melanoma inhibitory activity 2 was found as a MIA homologue via gene search. In contrast to MIA, it is specifically expressed in hepatocytes. Experiments showed a higher expression of MIA2 during liver diseases like fibrosis or cirrhosis, whereas MIA2 is downregulated in hepatocellular carcinoma (HCC). Because of these effects, MIA2 was characterized as a tumour suppressor and a potential marker of liver damage. We investigate MIA2 in vitro regarding its potential interaction partners, localization and molecular function. Furthermore, the function of this protein in vivo is analyzed via a MIA2 knockdown mouse model.
Plasma medicine is an innovative and emerging field combining plasma physics, life sciences and clinical medicine to use physical plasma for therapeutic applications. Possible applications of plasma in medicine are antiseptics of medical devices or body surfaces, treatment of wounds, skin or fibrotic diseases.
Our aim is to study the cellular mechanisms and molecular functions of plasma during wound repair and treatment of different tumors.

In cooperation with Prof. Dr.-Ing. Peter Haring Bolivar (University Siegen, Lehrstuhl Höchstfrequenztechnik und Quantenelektronik) a procedure to detect hybridized DNA strands without labelling has been developed. This method wil be applied to the development of the so-called DNA CHIP technology. Already, a patent emerged from this cooperation.
We are working on the development of 3D-printed tumor models that represent different aspects of tumorigenesis and tumor progression (as part of the DFG-funded Collaborative Research Center Transregio 225 (SFB TRR225, Biofabrication; Project C03, Z03)) or focus on the importance of biomechanics in the context of brain development or tumor metastasis to the brain (SFB 1540, EBM, Project C04). These models show improved transferability to the in vivo system compared to conventional cell culture models by incorporating extracellular matrix, stromal cells, and three-dimensionality. We ask basic scientific questions about the origin of quiescent tumor cells, a phenomenon called tumor dormancy, and cellular differentiation that can be answered by these innovative, controllable models.